Due to the fact that a relatively large area can be disinfected in a short time, dry-fog disinfection could have great potential to improve hygienic measures. However, there are few studies to date on the effectiveness and combinability of this technology with other devices. In this work, the disinfecting potential of the TBT dry fogging technology (TBT Desinfektion GmbH & Co. KG, Germany) and the corresponding disinfectant Defeat AR (Biofluid GmbH, Germany) was examined with regard to microbiological decontamination and potentially damaging effects on different devices.
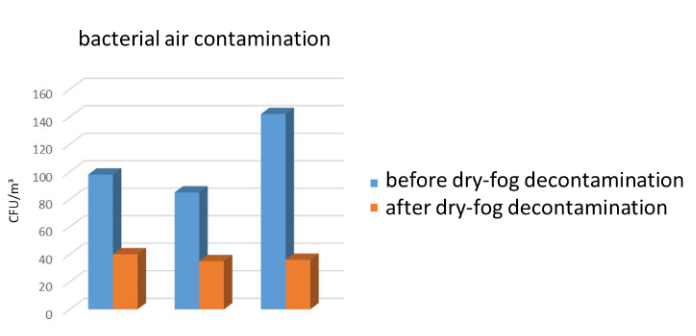
The air burden measurement did show a reduction of approx. 66% of the microbiological room contamination. Similarly, decontamination of test surfaces was shown to be effective at all measuring points we selected in the experimental setup. Depending on the accessibility of the surfaces, this effect was in the range of several log levels. Nevertheless, a first test run showed that some technical equipment was negatively affected by the process.
Technology for infection control during the pandemic. [2] It needs to be assessed whether these disinfection technologies are able to fulfill the requirements for hygienic measures in the medical field. On the other hand, especially in the clinical environment, a large number of sensitive technical equipment is present. Particularly in the case of actively ventilated devices, it cannot be ruled out that residues may remain in the devices as a result of fogging and lead to a defect.
A damaging effect on sensitive technical equipment by room decontamination should be checked in advance in order to exclude a disturbance of the equipment and thus disturb the monitoring and treatment of patients. In this paper, the potential of surface and room air decontamination of the TBT dry fogging technology (TBT Desinfektion GmbH & Co. KG, Germany) and the corresponding disinfectant Defeat AR (Biofluid GmbH, Germany) was tested by microbiological methods. Ventilated technical test samples were used to investigate the impact of the technology on the integrity of medical devices.